is salt solution homogeneous or heterogeneous
Some homogeneous mixtures are components of heterogeneous mixtures. Homogeneous mixtures have uniform composition and appearance throughoutA homogeneous mixture is also called a true solution.

Is Matter Around Us Pure School Help By Gunjan Pure Products School Help Homogeneous Mixture
Solutions are homogeneous mixtures that contain a solute dissolved in a solvent eg.

. An example of colloid in which dispersed phase is gas and dispersion medium is solid a pumice stone b whipped. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. In heterogeneous equilibrium substances are in different phases.
When Identifying Homogeneous and. A solution composed of matter that all exists in the same state. Mixtures can be homogeneous mixtures or heterogeneous mixtures.
The key difference between homogeneous and heterogeneous is that homogeneous materials and mixtures have the same uniform composition and properties throughout whereas heterogeneous materials and mixtures do not have either uniform composition or uniform properties. A homogeneous mixture looks like all one thing even though it is made up of different components. Sugar solution ocean water soft drinks etc.
A solution composed of different states of matter. Rubbing alcohol homogeneous 4. Homogeneous mixtures are also known as solutionsWhen you think of a solution you probably think of a liquid.
The state of a reaction in which the rates of the forward and reverse reactions are the same. There are 2 types of matter. For example you can make a homogeneous solution of sugar and water but if there are crystals in the solution it becomes a heterogeneous mixture.
Emulsions are homogeneous mixtures although they often become. Salt dissolved in water. Homogeneous mixture Solution.
The exception would be solutions that contain another phase of matter. Black coffee homogeneous 15. Heterogeneous solutions are solutions with non-uniform composition and.
OR classify as a mixture or pure substance. Yes a solution is a homogeneous mixture. Aluminum foil homogeneous 14.
These are called aqueous solutions denoted aq in which water acts as a solvent. Chemical solutions are usually homogeneous mixtures. Elements and compounds are types of pure substances.
Mixtures often contain a variety of substances and compounds and the components may be homogeneous or heterogeneous. Homogeneous mixture examples include dissolving things like salt sugar or food coloring into water. Salt and pepper form a heterogeneous mixture.
Italian salad dressing heterogeneous 11. Solid Homogeneous Mixture Examples. There are three families of mixtures.
Homogeneous and heterogeneous are two different words that we can distinguish by the context in. Any chemical solution or alloy is a homogeneous mixture. Heterogeneous mixtures consist of different substances.
Some mixtures appear homogeneous from a distance but are heterogeneous upon closer inspection. For example in the nucleation of ice from supercooled water droplets purifying the water to remove all or almost all impurities results in water droplets that freeze below around 35 C whereas water that contains impurities may freeze at 5 C or warmer. A Oil and vinegar salad dressing is a heterogeneous mixture because its composition is not uniform throughout.
Heterogeneous nucleation nucleation with the nucleus at a surface is much more common than homogeneous nucleation. Beach sand heterogeneous 6. An example of a homogeneous solution is a soft drink.
Often it is easy to confuse a homogeneous mixture with a pure substance because they are both uniform. Solutions have particles which are the size of atoms or molecules - too small to be seen. Sugar water homogeneous heterogeneous.
Mixtures can be either homogeneous or heterogeneous. Examples of solutions include sugar water and powdered drink mix in water while alloys include sterling silver and bronze. It is a a suspension b colloid c true solution d both a colloid and a true solution.
Solutions are homogeneous while suspensions and colloids are heterogeneous. A mixture in which constituents are distributed uniformly such as salt in water is called homogeneous whereas a mixture whose constituents are clearly separate from one another such as sand in water it is called heterogeneous. The steel legs of a classroom chair are made from an alloy a homogeneous mixture.
Chocolate chip cookies are a heterogeneous mixture. In chemistry these can be due to chemical reactions between the components. Pure air homogeneous 7.
Homogeneous and heterogeneous mixtures. For example a cup of coffee perfume cough syrup a solution of salt or sugar in water etc. A solution is a special type of mixture that is homogeneous where you cannot tell the difference between the components.
A colloid is a homogeneous solution with intermediate particle size between a solution and a suspension. A true solution is a homogeneous b heterogeneous c translucent d opaque. A homogeneous mixture is a mixture throughout the solution in which the composition is uniform.
Solutions suspensions and colloids. Other examples of homogeneous mixtures include air maple syrup gasoline and a solution of salt in water. Seawater is a liquid that looks clear.
In contrast when they cannot be mixed they are immisciblethey will form two separate layers called a heterogeneous solution. Classify the following as an element compound homogeneous mixture or heterogeneous mixture. Pure substances and mixtures.
For example salt is a heterogeneous mixture of sodium and chloride ions in water molecules. Chunky spaghetti sauce 8. Corn syrup homogeneous 12.
Homogeneous solutions are solutions with uniform composition and properties throughout the solution. Many common chemicals are homogeneous mixtures. If you take a bite from a cookie you may not get the same number of chips as you get in another bite.
Soda is considered a heterogeneous mixture. Another word for a homogeneous mixture is solutionThus a combination of salt and steel wool is a heterogeneous mixture because it is easy to see which particles of the matter are salt crystals and which are steel wool. Full fat milk heterogeneous 5.
When something dissolves it is broken down completely to the molecular level. A solution is also a special type of mixture that cannot be separated via mechanical means filtering screening etc. A solution is transparent blue.
In addition uniform mixture is another term for homogenous mixture. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves as a single substance. Examples include soil blood and sand.
The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample. The saltwater mentioned above is homogeneous due to the even distribution of the dissolved salt throughout the entire sample of saltwater. To be able to mix the molecules of both liquids have to be.
However many solids are also considered homogenous mixturesThere is a wide variety of solid homogeneous mixtures from naturally occurring materials like stone to synthetic plastics. Note that we can see through a homogeneous solution. For example bitumen is a homogeneous mixture that is a component of asphalt a heterogeneous mixture.
In most cases a solution has different properties than the two or more parts that went into making it. B A commercial sports drink is a homogeneous mixture because its composition is uniform throughout. Homogeneous and heterogeneous are.
Particle size distinguishes homogeneous solutions from other heterogeneous mixtures. It contains water sugar and carbon dioxide which forms bubbles. When the solvent is water it is called an aqueous solution.

Selina Concise Chemistry Class 6 Icse Solutions Chapter 5 Pure Substances And Mixtures Separation Of Mixtures Ncert Chemistry Class Chemistry Science Notes

A Scheme For Classifying Matter Teaching Chemistry Science Lessons Chemistry
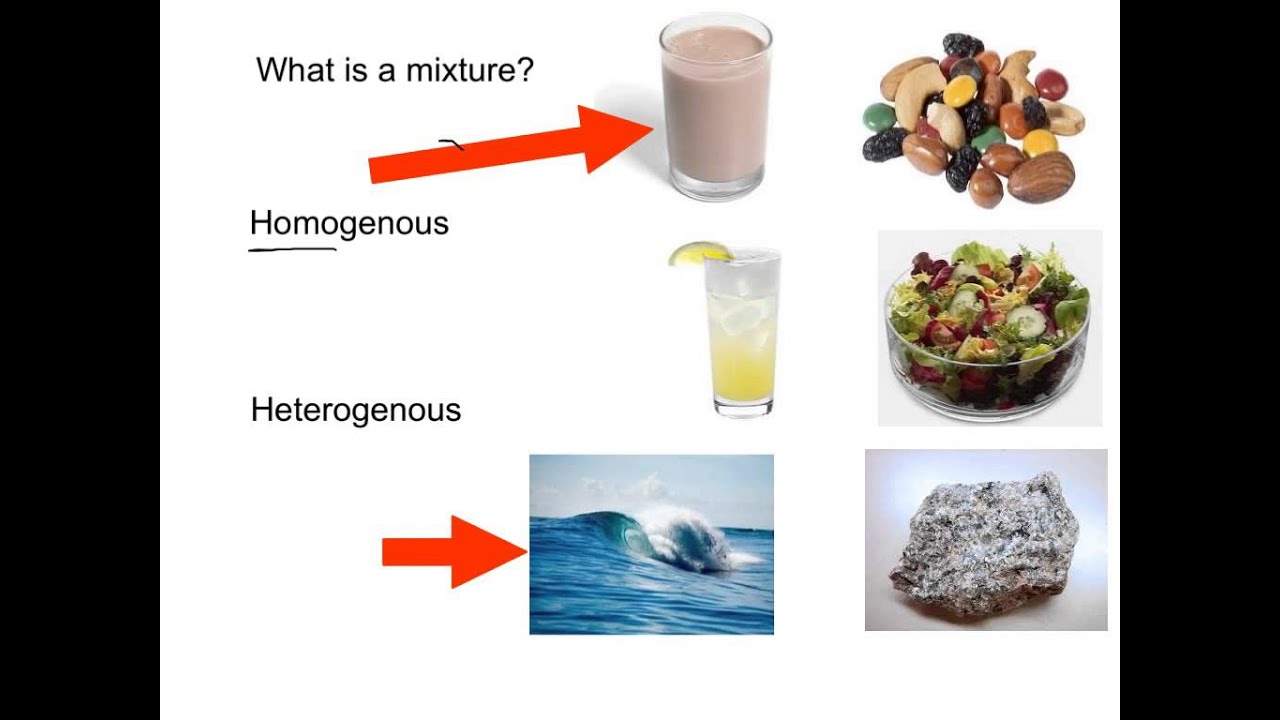
Pin On Middle School Chemistry

Elements Atoms And Molecules Organic Chemistry Books Science Lesson Plans Science Notes

Science Focus Topic 2 Notes Mixing And Dissolving Compounds And Mixtures Elements Compounds And Mixtures School Science Experiments

Pure Substances And Mixtures Pure Products Elements Compounds And Mixtures Compounds And Mixtures

Physical Science Lessons 5th Grade Science Middle School Science Experiments

Let S Do An Experiment Homogeneous And Heterogeneous Mixtures Heterogeneous Mixture 5th Grade Science Physical Changes Experiments

9 Differences Between Solute And Solvent Solute Vs Solvent Lewis Acids And Bases Solubility Solvent

Pin By Carrie Flanagan On School Stuff Chemical Science Matter Science Chemistry Classroom

Let S Mention The Difference Between A Solution And A Heterogeneous Mixture A Solution Is A Comb Chemical Science Solutions And Mixtures Heterogeneous Mixture

Mixtures And Pure Substances Heterogeneous Mixture Pure Products Mixtures

Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Middle School Science Resources

Pure Substance Easy Science Pure Products Easy Science Chemistry Experiments

Frank Icse Solutions For Class 9 Chemistry Elements Compounds And Mixtures A Plus Topper Separationofmixturesc Compounds And Mixtures Chemistry Solutions

Matter Pure Substance Elementcompound Mixture Homogeneous Solution Colloid Heterogeneous Suspension Solutions Cell Processes Forms Of Matter

Homogeneous And Heterogeneous Mixtures Examples Classification Of Matte In 2021 Heterogeneous Mixture Chemistry Organic Chemistry Tutor

Matter And Change Lab Stations Station Physical Science Station Activities

Komentar
Posting Komentar